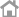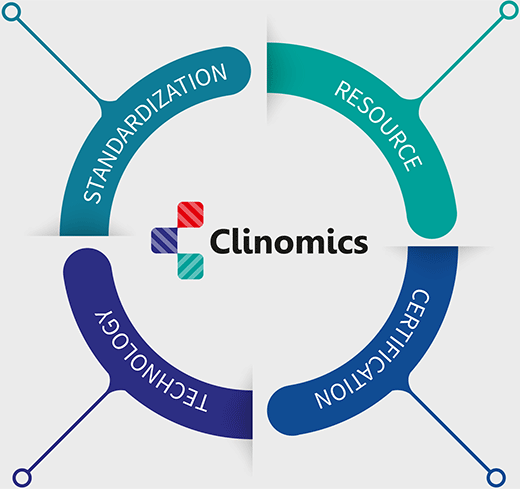
Standardization
Korean Standard Reference Genome (KOREF; Nationally certified)
Korean National Standard Reference Variome (KoVariome; Nationally certified)
The first Korean genome sequence and analysis
The first reference genomes for tiger, whale, and leopard
Resource
Genome-based biomedical information processing and high-quality human resources, long experiences and know-hows
More than 200 genome analysis systems/pipelines
Technology
Precise genome analysis algorithm
Increased genome analysis accuracy using Artificial Intelligence (AI)
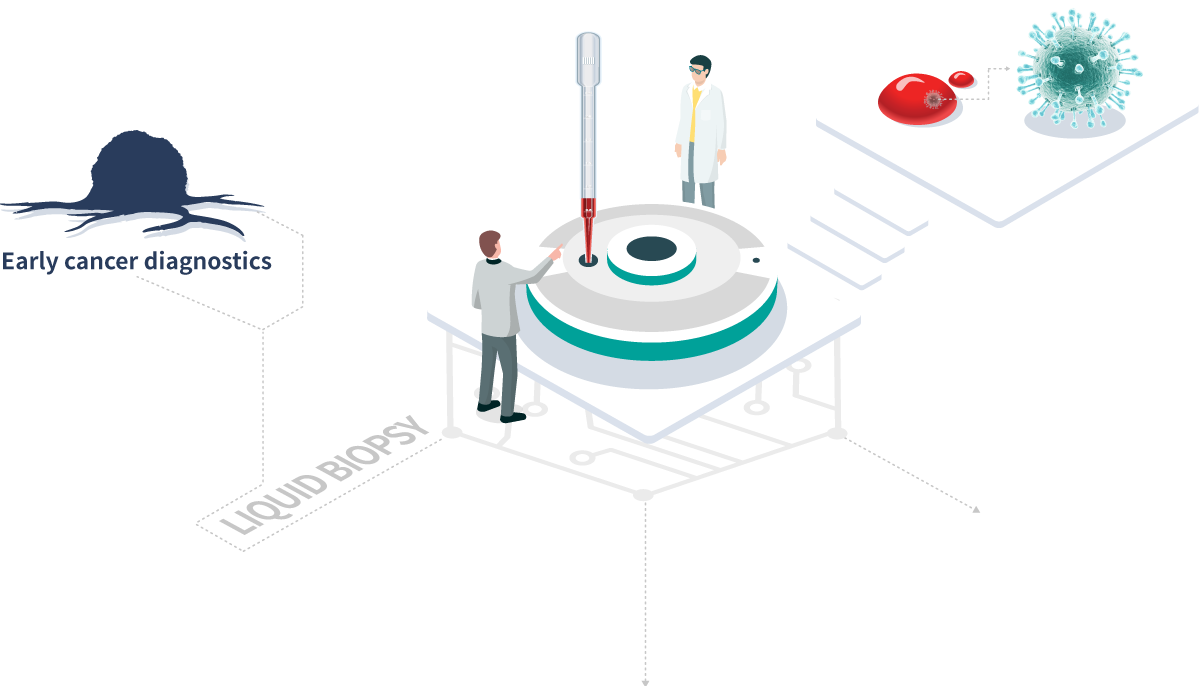
Commercialized high-efficiency liquid biopsy technology
Blood-based cancer genome analysis technology
Commercialized Asia’s first personal genome analysis service
Released high-speed automatic liquid biopsy device
Certification
CD-PRIME™ Class 1 medical device
Registered in FDA (US), CE (Europe)
Certified for GMP facility (ISO13485)
AmoyDx ROS1 selected as a target anticancer drug for lung cancer
Obtained a Class 3 for mutation kit from the Ministry of Food and Drug Safety